Sekce:
Daily overview
Selection from Decision-Making Practice - 4
Ensuring the Right to Full Reimbursement for Medicinal Products Listed in Annex No. 2
Pharmeca a.s.
03/26/2025
Sekce:
Focused on
Roles of Key Stakeholders in the Drug Reimbursement Process in the Czech Republic
The process of determining the reimbursement of medicinal products in the Czech Republic is complex and involves several key stakeholders who collectively influence the final decision. In our...
Pharmeca a.s.
03/21/2025
Sekce:
Daily overview
Selection from Decision-Making Practice - 3
Health Insurance Companies' Statement on the Unacceptability of Budget Impact.
Pharmeca a.s.
03/19/2025
Sekce:
Daily overview
Selection from Decision-Making Practice - 2
The State Institute for Drug Control questioned the cost-effectiveness presented in the administrative proceedings.
Pharmeca a.s.
03/12/2025
Sekce:
Daily overview
Selection from Decision-Making Practice
Multiple reimbursement levels for JAKi in reimbursement review.
Pharmeca a.s.
03/05/2025
Sekce:
Daily overview
EMA Launches New Platform to Monitor Medicine Shortages
The European Medicines Agency (EMA) has announced the launch of a new platform designed to routinely monitor shortages of centrally authorized medicines.
Pharmeca a.s.
01/31/2025
Sekce:
Data visualization
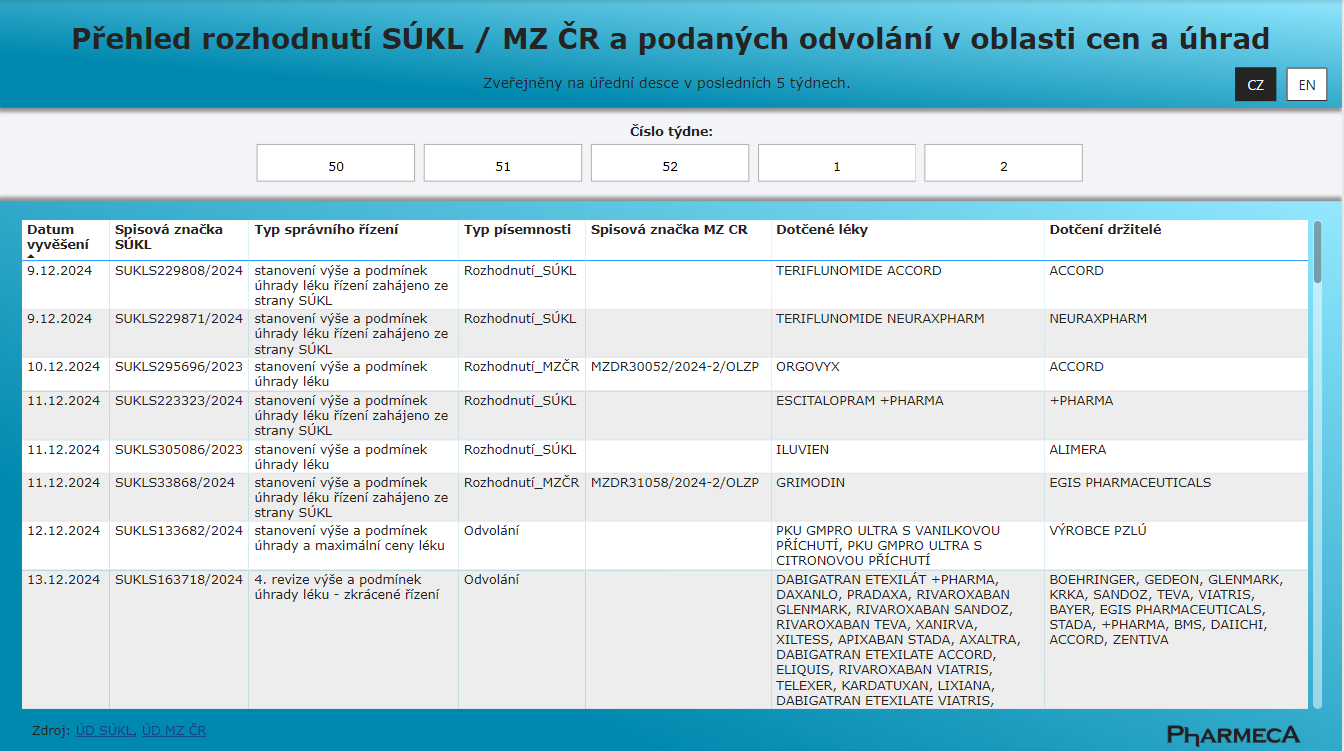
Decisions of the State Institute for Drug Control and the Ministry of Health of the Czech Republic in the Area of Pricing and Reimbursement
From January 1, 2025, Pharmeca a.s. offers an overview of SÚKL and Ministry of Health decisions on pricing and reimbursement.

Pharmeca a.s.
01/08/2025
Sekce:
Focused on
EMA Launches New Platform to Monitor Medicine Shortages
The European Medicines Agency (EMA) has announced the launch of a new platform designed to routinely monitor shortages of centrally authorized medicines.
Pharmeca a.s.
12/16/2024

