Sekce:
Focused on
Impacts of the New SUKL Decision-Making Practice on the Reimbursement of Innovative Medicines
The pharmaceutical market in the Czech Republic is undergoing a gradual shift in decision-making practices, which may significantly impact pricing and reimbursement regulations as well as the...
03/07/2025
Sekce:
Daily overview
Selection from Decision-Making Practice
Multiple reimbursement levels for JAKi in reimbursement review.
Pharmeca a.s.
03/05/2025
Sekce:
Daily overview
Opinion of the Ministry of Health of the Czech Republic on the impact on the system when setting prices and reimbursements.
The Ministry of Health of the Czech Republic has published an opinion on the procedure for assessing the financial impact on the health insurance system when determining or changing the amount and...
MZ ČR
02/24/2025
Sekce:
Daily overview
EMA Launches New Platform to Monitor Medicine Shortages
The European Medicines Agency (EMA) has announced the launch of a new platform designed to routinely monitor shortages of centrally authorized medicines.
Pharmeca a.s.
01/31/2025
Sekce:
Data visualization
Decisions of the State Institute for Drug Control and the Ministry of Health of the Czech Republic in the Area of Pricing and Reimbursement
From January 1, 2025, Pharmeca a.s. offers an overview of SÚKL and Ministry of Health decisions on pricing and reimbursement.
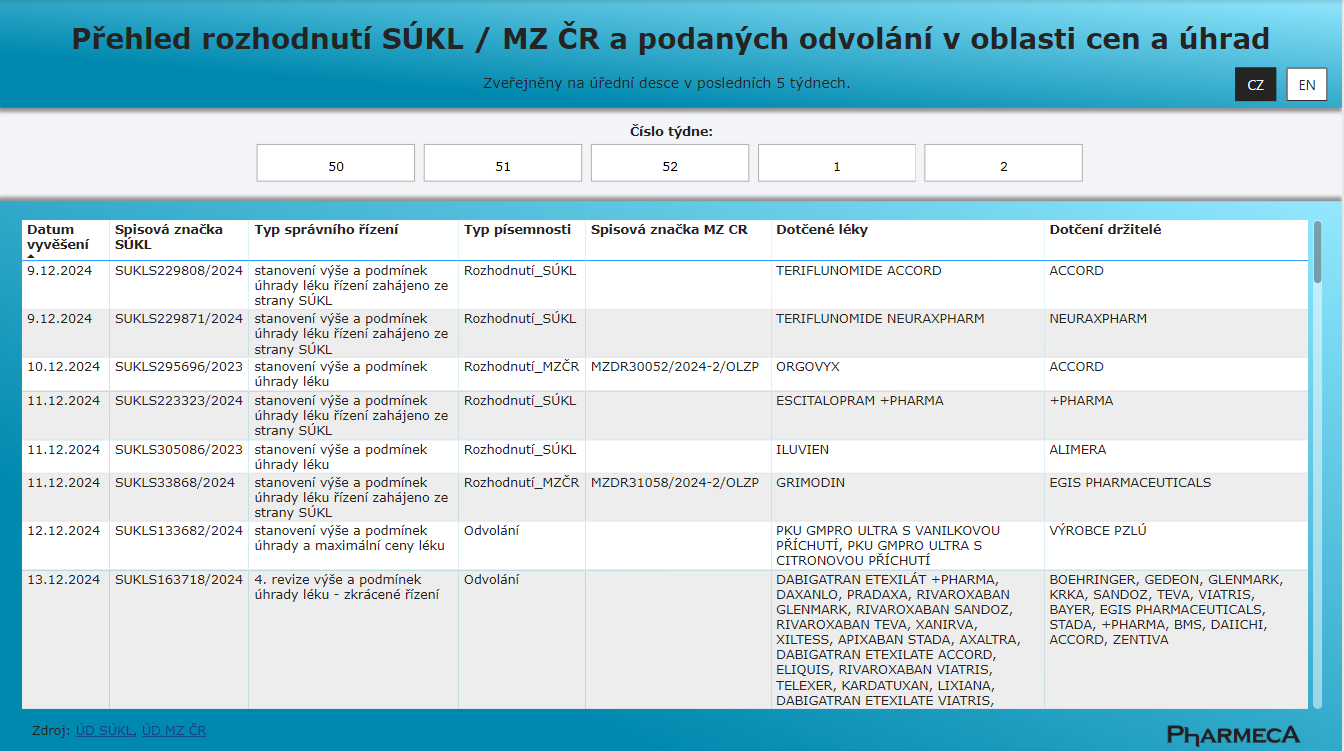

Pharmeca a.s.
01/08/2025
Sekce:
Focused on
EMA Launches New Platform to Monitor Medicine Shortages
The European Medicines Agency (EMA) has announced the launch of a new platform designed to routinely monitor shortages of centrally authorized medicines.
Pharmeca a.s.
12/16/2024
Sekce:
Focused on
Medical Devices and Medicines in Slovakia to Have Lower VAT from 2025
From January 1, 2025, Slovakia will introduce a significant change in its tax policy. This step is part of a broader tax reform aimed at improving access to essential goods for the population while...
Pharmeca
12/13/2024
Sekce:
Focused on
European Health Data Space 2024 update
The European Health Data Space (EHDS) has advanced significantly since the initial concept of a digital transformation in healthcare across all EU member states.
Pharmeca
11/18/2024
Sekce:
Focused on
Save the date: EMA organizuje Information Day for CTIS 17. října 2024
Informační systém klinických studií (CTIS) – pokračující proces přechodu
Pharmeca a.s.
10/07/2024
Sekce:
Focused on
Clinical Trials Information System (CTIS) - present and future
CTIS podporuje interakce mezi sponzory klinických studií (výzkumníky nebo společnostmi, které provádějí klinickou studii a shromažďují a analyzují data) a regulačními orgány v členských státech EU...
Pharmeca
03/08/2024
Sekce:
Focused on
Change in VAT Rates for Medicines
Based on the amendment to Act No. 235/2004 Coll., on Value Added Tax, as of January 1, 2024, there has been a change in VAT rates, particularly impacting the increase in prices and reimbursements...
Pharmeca a.s.
02/27/2024
Sekce:
Daily overview
A decision of the Ministry of Health of the CR under the no. MZDR 20570/2021-2/OLZP
A decision of the Ministry of Health regarding the appeal of the appelant sanofi-aventis, s.r.o. - the appeal is dismissed and the decision under appeal is upheld.
MZCR
03/24/2023
Sekce:
Daily overview
A decision of the Ministry of Health of the CR under the no. MZDR 23086/2021-2/OLZP
A decision of the Ministry of Health regarding the appeal of the appelant Egis Pharmaceuticals PLC - the appeal is dismissed and the decision under appeal is upheld.
MZCR
03/20/2023
Sekce:
Daily overview
A decision of the Ministry of Health of the CR under the no. MZDR 24311/2021-2/OLZP
A decision of the Ministry of Health regarding the appeal of the appelant Egis Pharmaceuticals PLC - the appeal is dismissed and the decision under appeal is upheld.
MZCR
03/20/2023
Sekce:
Daily overview
A decision of the Ministry of Health of the CR under the no. MZDR 29481/2022-2/OLZP
A decision of the Ministry of Health regarding the appeal of the appelant Union of Health Insurance Companies - the decision is reversed and the issue is referred back for reappraisal.
MZCR
03/01/2023
Sekce:
Daily overview
A decision of the Ministry of Health of the CR under the no. MZDR 19039/2021-2/OLZP
A decision of the Ministry of Health regarding the appeal of the appelant Otsuka Pharmaceutical Netherlands B. V. - the appeal is dismissed and the decision under appeal is upheld.
MZCR
02/20/2023

