Sekce:
Focused on
Orphan Medicinal Products from the Perspective of Decision-Making Practice
In 2025, SÚKL issued a total of 12 final decisions in proceedings concerning the determination of price and reimbursement for orphan medicinal products, based on binding opinions of the Ministry of...
Pharmeca a.s.
02/27/2026
Sekce:
Daily overview
Selection from Decision-Making Practice - 3/2026
Determination of Reimbursement to Ensure Availability.
Pharmeca a.s.
02/18/2026
Sekce:
Daily overview
Possible Service Outages of Verso and DSŘ
SÚKL warns of possible limitations affecting remote access to case files via the Verso application and automated access to documents of administrative proceedings in CAU (DSŘ).
SÚKL
02/09/2026
Sekce:
Daily overview
Selection from Decision-Making Practice - 2/2026
Criteria Assessed for the First Similar Medicinal Product.
Pharmeca a.s.
02/04/2026
Sekce:
Daily overview
Update of Guideline CAU-08
SÚKL has published an updated version of guideline CAU-08 - Requirements on the structure of technical documentation to be submitted along with applications and on the structure of opinions of...
SÚKL
02/02/2026
Sekce:
Focused on
Exchange Rates for Price Referencing after the Amendment
The amendment to the Public Health Insurance Act and its implementing decrees has introduced changes, effective from the beginning of the year, also in the way exchange rates are applied for price...
Pharmeca a.s.
01/28/2026
Sekce:
Data visualization
Exchange Rates for Price Referencing at a Click
The key information for conducting price referencing in administrative procedures for drug pricing and reimbursement is the average exchange rate from the previous quarter. Now, you can easily...
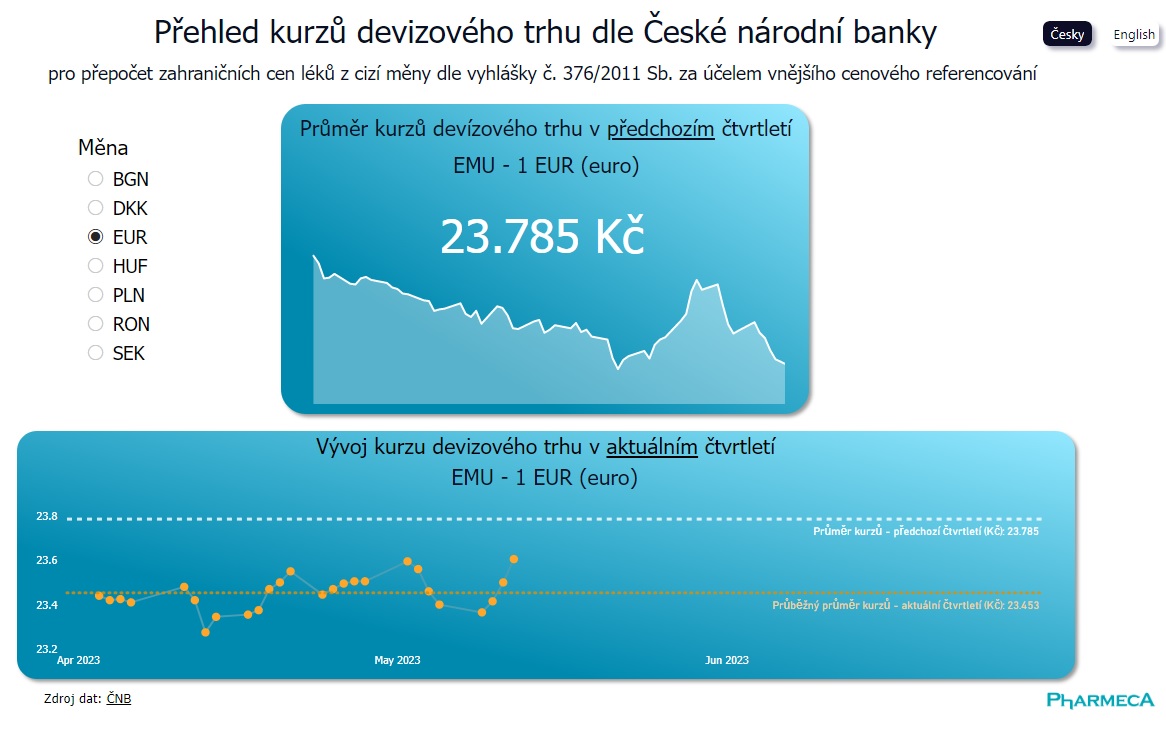

Pharmeca a.s.
01/28/2026
Sekce:
Daily overview
Update of Guideline CAU-08
SÚKL has published an updated version of guideline CAU-08 - Requirements on the structure of technical documentation to be submitted along with applications and on the structure of opinions of...
SÚKL
01/19/2026
Sekce:
Daily overview
Update of Guideline CAU-08
SÚKL has published an updated version of guideline CAU-08 - Requirements on the structure of technical documentation to be submitted along with applications and on the structure of opinions of...
SÚKL
01/16/2026
Sekce:
Daily overview
Selection from Decision-Making Practice - 1/2026
Ensuring a Fully Reimbursed Medicinal Product – Diseases of the Same Type
Pharmeca a.s.
01/14/2026
Sekce:
Daily overview
Update of Guideline CAU-08
SÚKL has published an updated version of guideline CAU-08 - Requirements on the structure of technical documentation to be submitted along with applications and on the structure of opinions of...
SÚKL
01/02/2026
Sekce:
Daily overview
Update of Guideline CAU-08
SÚKL has published an updated version of guideline CAU-08 - Requirements on the structure of technical documentation to be submitted along with applications and on the structure of opinions of...
SÚKL
01/02/2026
Sekce:
Daily overview
Update of Guideline CAU-08
SÚKL has published an updated version of guideline CAU-08 - Requirements on the structure of technical documentation to be submitted along with applications and on the structure of opinions of...
SÚKL
01/02/2026
Sekce:
Daily overview
Update of Guideline CAU-08
SÚKL has published an updated version of guideline CAU-08 - Requirements on the structure of technical documentation to be submitted along with applications and on the structure of opinions of...
SÚKL
12/31/2025
Sekce:
Focused on
Czech legislation
An up-to-date overview of selected Czech legal regulations regarding medicines and medical devices.
Pharmeca
12/30/2025


